Patients can start and stay on Humalog U-200 KwikPen as their insulin needs increase*
*Option for patients who require >20 units of mealtime insulin/day.
Bioequivalence – with half the volume
Humalog U-200 is bioequivalent* to Humalog U-100; therefore you should expect similar efficacy and safety as Humalog U-1001. Humalog U-200 KwikPen delivers the same dose of insulin in half the liquid volume.1
Humalog U-200 was bioequivalent* to Humalog U-100 after subcutaneous administration of a single 20-unit dose in healthy subjects. Time to maximum concentration was also similar between formulations.

This line graph presents the pharmacokinetics of Humalog (insulin lispro) U-200 (200 units per milliliter) and Humalog (insulin lispro) U-100 (100 units per milliliter). The Y-axis shows mean free serum immunoreactive insulin concentrations ranging from 0 to 1000 picomoles per liter. The X-axis expresses time in hours after insulin lispro administration, ranging from 0 to 6 hours. Peak serum insulin concentration for U-100 was 839 picomoles per liter (standard error equals 34), occurring 1 hour after insulin administration. Peak serum insulin concentration for U-200 was 745 picomoles per liter (standard error equals 33), also occurring 1 hour after insulin administration.
*As determined by area under the curve and maximum concentration.

This line graph presents the pharmacodynamics of Humalog (insulin lispro) U-200 (200 units per milliliter), 20 units administered subcutaneously (sc) and Humalog (insulin lispro) U-100 (100 units per milliliter), 20 units administered subcutaneously (sc). The Y-axis shows the mean glucose infusion rate, with values ranging from 0 to 600 milligrams per minute. The X-axis expresses time in hours from insulin lispro dose administration, ranging from 0 to 8 hours. Insulin lispro U-100 had a peak mean glucose infusion rate of 418.4 milligrams per minute at hour 3.0, while insulin lispro U-200 had a peak mean glucose infusion rate of 415.2 milligrams per minute at hour 2.7.
Select Important Safety Information
Instruct patients to always check the insulin label before each injection to avoid medication errors. Do NOT transfer Humalog U-200 from the Humalog KwikPen to a syringe as overdose and severe hypoglycemia can occur.
Dosing Volume
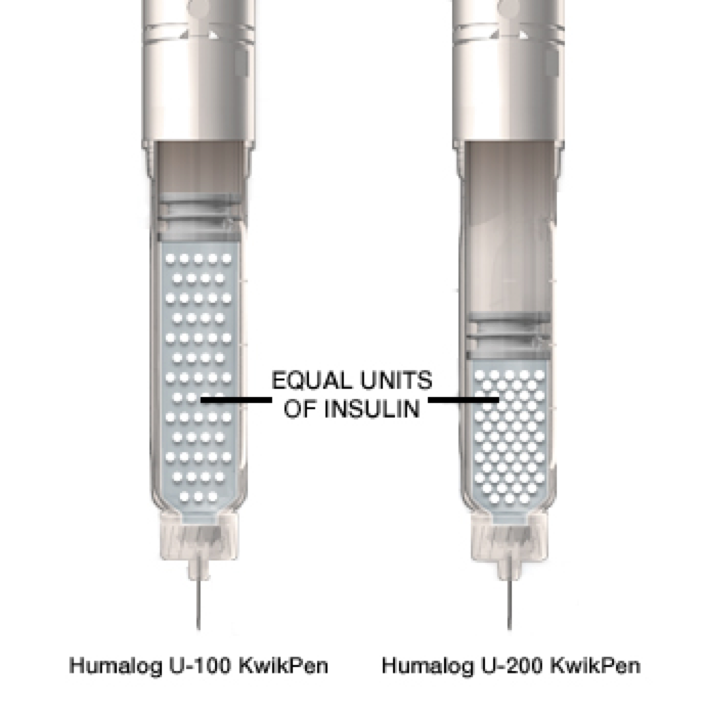
Delivers the same dose in half the liquid volume of Humalog U-100 KwikPen
Humalog 200 units/mL is twice the concentration of Humalog U-100 mealtime, rapid-acting insulin.
References: 1. Humalog [Prescribing Information]. Indianapolis, IN: Lilly USA, LLC; 2015. 2. Data on file, Lilly Research Laboratories: HI20150526A.
INDICATIONS
Humalog and Insulin Lispro Injection are rapid-acting insulin analogs indicated to improve glycemic control in adult and pediatric patients with diabetes mellitus. Humalog Mix75/25 and Humalog Mix50/50, and Insulin Lispro Protamine and Insulin Lispro Injectable Suspension Mix75/25 are mixtures of intermediate-acting and rapid-acting human insulin analogs indicated to improve glycemic control in patients with diabetes mellitus.
Limitations of Use: The proportions of rapid-acting and intermediate-acting insulins in Humalog Mix75/25 and Humalog Mix50/50, and Insulin Lispro Protamine and Insulin Lispro Injectable Suspension Mix75/25 are fixed and do not allow for basal versus prandial dose adjustments.