Device Options


Humalog KwikPen®
- Humalog KwikPen is a small, lightweight, prefilled insulin pen for patients with type 2 or type 1 diabetes who want a portable device. Humalog KwikPen must be stored outside the refrigerator after the first use and taken just about anywhere. Patients can carry it in their purse, backpack, or pocket because it's the size of a marker.*
- Unopened Humalog should be stored in a refrigerator at 36°F to 46°F (2°C to 8°C) and can be used until the expiration date on the carton or label.
- In-use Humalog KwikPen should be stored at room temperature, below 86°F (30°C) and must be used within 28 days or be discarded, even if it still contains Humalog.
*Store at room temperature below 86°F.
Select Important Safety Information
Never share a Humalog or Insulin Lispro Injection prefilled pen, cartridge, reusable pen compatible with Lilly 3 mL cartridges, or syringe between patients as it poses a risk for transmission of bloodborne pathogens.
Instruct patients to always check the insulin label before each injection to avoid medication errors. Do NOT transfer Humalog U-200 from the Humalog KwikPen to a syringe as overdose and severe hypoglycemia can occur.
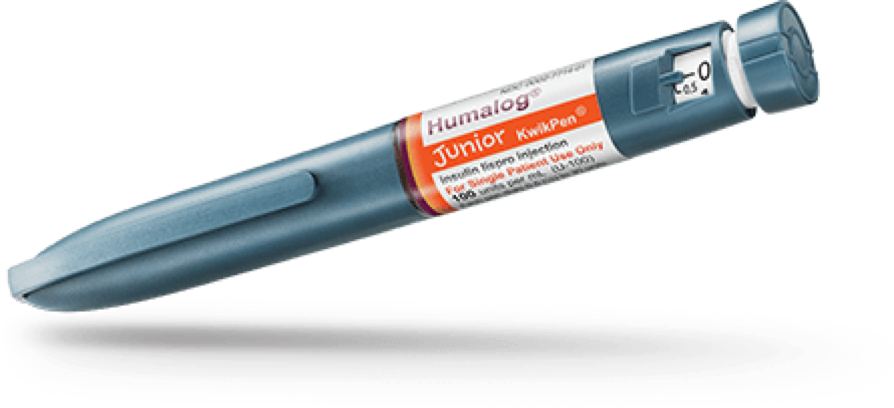
Humalog Junior KwikPen®
- Humalog Junior KwikPen is an insulin pen that offers the combined benefits of a rapid acting insulin in a prefilled disposable pen and half-unit dosing.
- In-use Humalog KwikPen should be stored at room temperature, below 86ºF (30ºC) and must be used within 28 days or be discarded, even if it still contains Humalog.
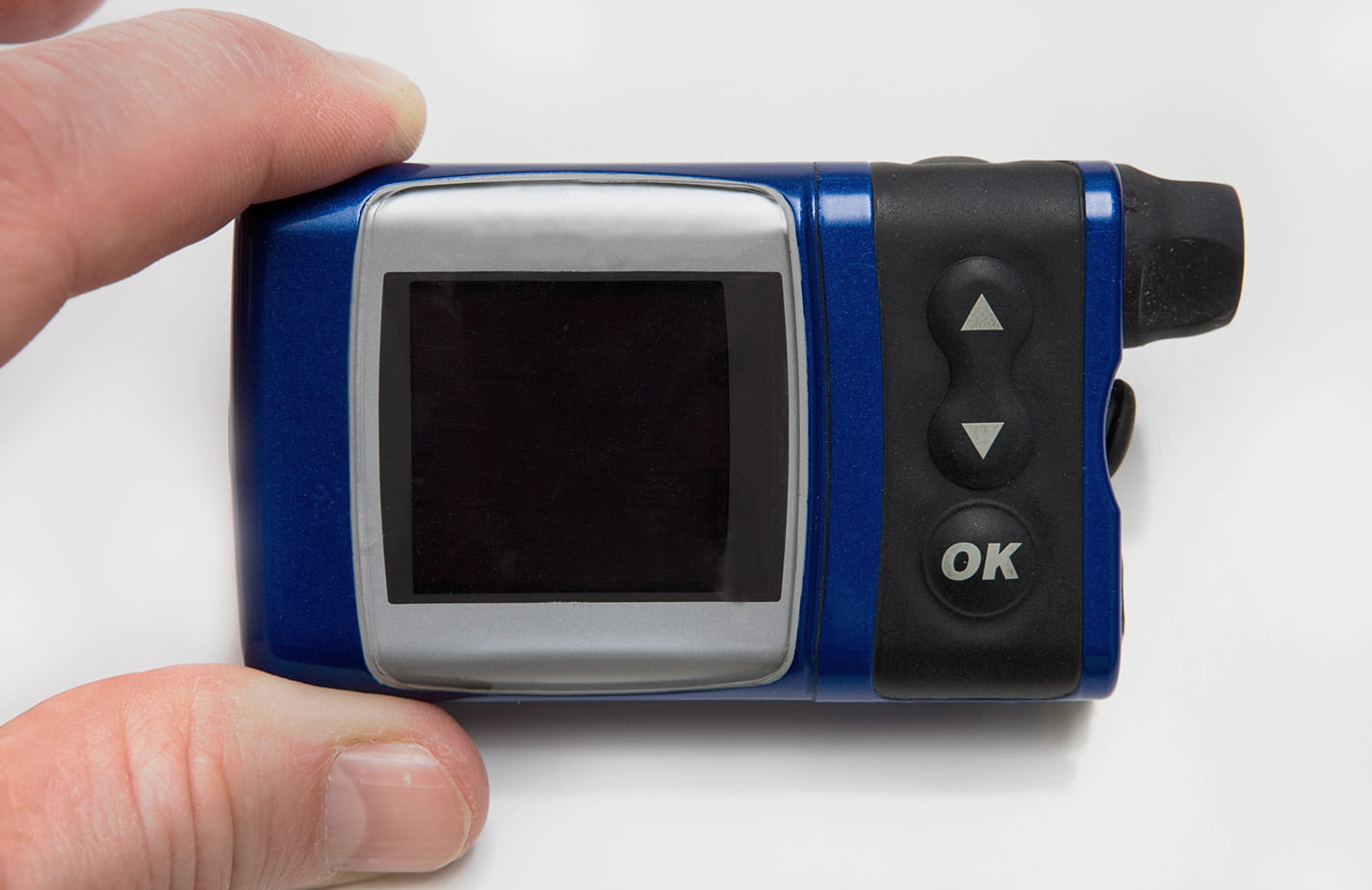
Use of Humalog U-100 in an Insulin Pump
For some people, an insulin pump can help improve diabetes management and make it more flexible. A pump may be a good option for someone who:
- Regularly tests their blood sugar
- Is active and has an inconsistent meal and physical activity schedule
- Has demonstrated the ability to manage his/her diabetes but doesn’t want to draw attention to it
Select Important Safety Information
Insulin pump or infusion set malfunction or degradation of the insulin in the pump reservoir can rapidly result in hyperglycemia and ketoacidosis. Patients should be instructed to carry alternate insulin therapy in case of pump failure.
Catheter occlusions and infusion-site reactions have been reported in patients receiving Humalog U-100 or Insulin Lispro Injection as a continuous subcutaneous infusion.
Humalog U-100 or Insulin Lispro Injection in an external insulin pump is approved for use in adults and children with diabetes mellitus. Humalog U-100 and Insulin Lispro Injection have not been studied in children with type 2 diabetes or in children with type 1 diabetes less than 3 years of age.
When used in a pump, do not mix Humalog U-100 with any other insulin or liquid. Do not use Humalog Mix75/25, Humalog Mix50/50, or Humalog U-200 in a pump.
INDICATIONS
Humalog and Insulin Lispro Injection are rapid-acting insulin analogs indicated to improve glycemic control in adult and pediatric patients with diabetes mellitus. Humalog Mix75/25 and Humalog Mix50/50, and Insulin Lispro Protamine and Insulin Lispro Injectable Suspension Mix75/25 are mixtures of intermediate-acting and rapid-acting human insulin analogs indicated to improve glycemic control in patients with diabetes mellitus.
Limitations of Use: The proportions of rapid-acting and intermediate-acting insulins in Humalog Mix75/25 and Humalog Mix50/50, and Insulin Lispro Protamine and Insulin Lispro Injectable Suspension Mix75/25 are fixed and do not allow for basal versus prandial dose adjustments.